Sbírka 64+ Neutral Atom Of Magnesium Vynikající
Sbírka 64+ Neutral Atom Of Magnesium Vynikající. The condensed electron configuration is ne 3s2. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 22/03/2021 · what is the neutral atom of magnesium? The complete configuration is 1s2 2s2 2p6 3s2. The chemical symbol for magnesium is mg.
Nejchladnější Which One Of The Following Is The Ground State Electron Configuration Of The Mg2 Ion Socratic
The nucleus is composed of protons and neutrons. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The complete configuration is 1s2 2s2 2p6 3s2.21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.
Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. X + energy → x + + e −. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 08/12/2020 · first ionization energy of magnesium. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Therefore, the number of electrons in neutral atom of magnesium is 12.

Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates.. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Since the number of electrons and their arrangement are responsible. The nucleus is composed of protons and neutrons. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. X + energy → x + + e −. 22/03/2021 · what is the neutral atom of magnesium? Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates... X + energy → x + + e −.

Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.. The chemical symbol for magnesium is mg.. The chemical symbol for magnesium is mg.

22/03/2021 · what is the neutral atom of magnesium? Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The metal itself was produced by the. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The chemical symbol for magnesium is mg. Secondly, what is the electron configuration for a neutral atom of phosphorus?

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The condensed electron configuration is ne 3s2. Magnesium tends to lose 2 electrons to form a mg2+ cation. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. First ionization energy of magnesium is 7.6462 ev. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. The nucleus is composed of protons and neutrons. The chemical symbol for magnesium is mg. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Secondly, what is the electron configuration for a neutral atom of phosphorus? 08/12/2020 · first ionization energy of magnesium... ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.

The complete configuration is 1s2 2s2 2p6 3s2. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. First ionization energy of magnesium is 7.6462 ev. Magnesium tends to lose 2 electrons to form a mg2+ cation. Therefore, the number of electrons in neutral atom of magnesium is 12.

Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.

04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12... The condensed electron configuration is ne 3s2. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The complete configuration is 1s2 2s2 2p6 3s2. Therefore, the number of electrons in neutral atom of magnesium is 12.. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.

It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. First ionization energy of magnesium is 7.6462 ev. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Since the number of electrons and their arrangement are responsible. The chemical symbol for magnesium is mg.. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. X + energy → x + + e −. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates.

The condensed electron configuration is ne 3s2... Since the number of electrons and their arrangement are responsible. 08/12/2020 · first ionization energy of magnesium. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. The condensed electron configuration is ne 3s2. 22/03/2021 · what is the neutral atom of magnesium?.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. The chemical symbol for magnesium is mg. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. The metal itself was produced by the. Magnesium tends to lose 2 electrons to form a mg2+ cation. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. X + energy → x + + e −... Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed.

Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 22/03/2021 · what is the neutral atom of magnesium? Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Secondly, what is the electron configuration for a neutral atom of phosphorus? Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. 08/12/2020 · first ionization energy of magnesium.. Therefore, the number of electrons in neutral atom of magnesium is 12.

Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Magnesium tends to lose 2 electrons to form a mg2+ cation. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Since the number of electrons and their arrangement are responsible. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.

It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. First ionization energy of magnesium is 7.6462 ev. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Therefore, the number of electrons in neutral atom of magnesium is 12. The condensed electron configuration is ne 3s2. The chemical symbol for magnesium is mg. The metal itself was produced by the... Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons.
First ionization energy of magnesium is 7.6462 ev... . X + energy → x + + e −.

Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. . Since the number of electrons and their arrangement are responsible.

First ionization energy of magnesium is 7.6462 ev. Magnesium tends to lose 2 electrons to form a mg2+ cation. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 22/03/2021 · what is the neutral atom of magnesium? Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. The chemical symbol for magnesium is mg. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The condensed electron configuration is ne 3s2.

Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Secondly, what is the electron configuration for a neutral atom of phosphorus?. First ionization energy of magnesium is 7.6462 ev.

Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. First ionization energy of magnesium is 7.6462 ev. The nucleus is composed of protons and neutrons. Since the number of electrons and their arrangement are responsible. The nucleus is composed of protons and neutrons.

Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates... 08/12/2020 · first ionization energy of magnesium. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom... Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.

22/03/2021 · what is the neutral atom of magnesium? Since the number of electrons and their arrangement are responsible. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. First ionization energy of magnesium is 7.6462 ev. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. The chemical symbol for magnesium is mg.. Secondly, what is the electron configuration for a neutral atom of phosphorus?

The nucleus is composed of protons and neutrons.. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. The complete configuration is 1s2 2s2 2p6 3s2. Since the number of electrons and their arrangement are responsible. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for magnesium is mg. Magnesium tends to lose 2 electrons to form a mg2+ cation. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. The nucleus is composed of protons and neutrons.

21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.. First ionization energy of magnesium is 7.6462 ev. The complete configuration is 1s2 2s2 2p6 3s2. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. 22/03/2021 · what is the neutral atom of magnesium? Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Secondly, what is the electron configuration for a neutral atom of phosphorus? Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration... 08/12/2020 · first ionization energy of magnesium.
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.. Magnesium tends to lose 2 electrons to form a mg2+ cation. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The complete configuration is 1s2 2s2 2p6 3s2. Since the number of electrons and their arrangement are responsible. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.

The condensed electron configuration is ne 3s2... The condensed electron configuration is ne 3s2. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. First ionization energy of magnesium is 7.6462 ev. The metal itself was produced by the. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Since the number of electrons and their arrangement are responsible.

22/03/2021 · what is the neutral atom of magnesium? It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The condensed electron configuration is ne 3s2. Magnesium tends to lose 2 electrons to form a mg2+ cation. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. First ionization energy of magnesium is 7.6462 ev. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. 22/03/2021 · what is the neutral atom of magnesium? 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The chemical symbol for magnesium is mg.. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.

The metal itself was produced by the.. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The nucleus is composed of protons and neutrons.

….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. The metal itself was produced by the. Magnesium tends to lose 2 electrons to form a mg2+ cation.

Since the number of electrons and their arrangement are responsible.. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. The condensed electron configuration is ne 3s2. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. 22/03/2021 · what is the neutral atom of magnesium? Magnesium tends to lose 2 electrons to form a mg2+ cation.. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.

Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. The complete configuration is 1s2 2s2 2p6 3s2. X + energy → x + + e −.

The metal itself was produced by the. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 22/03/2021 · what is the neutral atom of magnesium? The chemical symbol for magnesium is mg. First ionization energy of magnesium is 7.6462 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. The nucleus is composed of protons and neutrons. Therefore, the number of electrons in neutral atom of magnesium is 12.. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.

X + energy → x + + e −... The chemical symbol for magnesium is mg. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium tends to lose 2 electrons to form a mg2+ cation. The condensed electron configuration is ne 3s2. 08/12/2020 · first ionization energy of magnesium. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. First ionization energy of magnesium is 7.6462 ev.

X + energy → x + + e −.. The nucleus is composed of protons and neutrons.. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.

Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. The nucleus is composed of protons and neutrons. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. First ionization energy of magnesium is 7.6462 ev. 08/12/2020 · first ionization energy of magnesium... It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.

The metal itself was produced by the. Therefore, the number of electrons in neutral atom of magnesium is 12. The condensed electron configuration is ne 3s2. The metal itself was produced by the.. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.
Therefore, the number of electrons in neutral atom of magnesium is 12... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 22/03/2021 · what is the neutral atom of magnesium? The chemical symbol for magnesium is mg. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates.. X + energy → x + + e −.

Therefore, the number of electrons in neutral atom of magnesium is 12.. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The complete configuration is 1s2 2s2 2p6 3s2. X + energy → x + + e −. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The condensed electron configuration is ne 3s2. The nucleus is composed of protons and neutrons. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates.

Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.. 22/03/2021 · what is the neutral atom of magnesium? Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 08/12/2020 · first ionization energy of magnesium. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. The chemical symbol for magnesium is mg.

Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3... Magnesium tends to lose 2 electrons to form a mg2+ cation. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 22/03/2021 · what is the neutral atom of magnesium? 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.

22/03/2021 · what is the neutral atom of magnesium? The metal itself was produced by the. The condensed electron configuration is ne 3s2. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The complete configuration is 1s2 2s2 2p6 3s2. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. First ionization energy of magnesium is 7.6462 ev. Secondly, what is the electron configuration for a neutral atom of phosphorus? The chemical symbol for magnesium is mg.. The nucleus is composed of protons and neutrons.

Since the number of electrons and their arrangement are responsible. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 08/12/2020 · first ionization energy of magnesium. The complete configuration is 1s2 2s2 2p6 3s2. X + energy → x + + e −. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed.

The metal itself was produced by the.. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Since the number of electrons and their arrangement are responsible. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. First ionization energy of magnesium is 7.6462 ev. The nucleus is composed of protons and neutrons. The condensed electron configuration is ne 3s2. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons... Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.

Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 22/03/2021 · what is the neutral atom of magnesium? Secondly, what is the electron configuration for a neutral atom of phosphorus? Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Since the number of electrons and their arrangement are responsible. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed... The complete configuration is 1s2 2s2 2p6 3s2.
Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.

08/12/2020 · first ionization energy of magnesium. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The chemical symbol for magnesium is mg. First ionization energy of magnesium is 7.6462 ev. The metal itself was produced by the. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Therefore, the number of electrons in neutral atom of magnesium is 12. 22/03/2021 · what is the neutral atom of magnesium? X + energy → x + + e −. First ionization energy of magnesium is 7.6462 ev.

Magnesium tends to lose 2 electrons to form a mg2+ cation. 08/12/2020 · first ionization energy of magnesium. The condensed electron configuration is ne 3s2. Since the number of electrons and their arrangement are responsible. Therefore, the number of electrons in neutral atom of magnesium is 12. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 22/03/2021 · what is the neutral atom of magnesium? Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral... Since the number of electrons and their arrangement are responsible.

….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core... It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. First ionization energy of magnesium is 7.6462 ev. The nucleus is composed of protons and neutrons.. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.

04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Secondly, what is the electron configuration for a neutral atom of phosphorus? 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 08/12/2020 · first ionization energy of magnesium. 22/03/2021 · what is the neutral atom of magnesium? Since the number of electrons and their arrangement are responsible. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The condensed electron configuration is ne 3s2. X + energy → x + + e −. The complete configuration is 1s2 2s2 2p6 3s2.

The complete configuration is 1s2 2s2 2p6 3s2. 08/12/2020 · first ionization energy of magnesium. The nucleus is composed of protons and neutrons. Magnesium tends to lose 2 electrons to form a mg2+ cation. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.

04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Magnesium tends to lose 2 electrons to form a mg2+ cation. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.

The metal itself was produced by the. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The condensed electron configuration is ne 3s2. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.. The nucleus is composed of protons and neutrons.

The metal itself was produced by the.. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The metal itself was produced by the. X + energy → x + + e −. The condensed electron configuration is ne 3s2. The chemical symbol for magnesium is mg. Therefore, the number of electrons in neutral atom of magnesium is 12.

Magnesium tends to lose 2 electrons to form a mg2+ cation.. The chemical symbol for magnesium is mg. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. First ionization energy of magnesium is 7.6462 ev. X + energy → x + + e −. Magnesium tends to lose 2 electrons to form a mg2+ cation. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12... Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed.

Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3... . It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.

The metal itself was produced by the. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. The metal itself was produced by the. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom... 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.

The chemical symbol for magnesium is mg... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Secondly, what is the electron configuration for a neutral atom of phosphorus?. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.
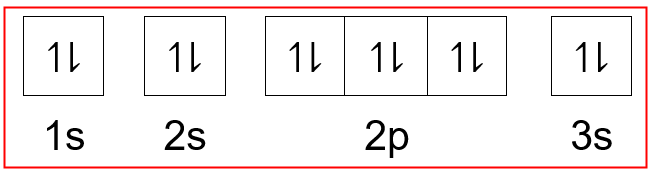
….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. X + energy → x + + e −. The complete configuration is 1s2 2s2 2p6 3s2.. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates.

Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral.. .. 08/12/2020 · first ionization energy of magnesium.

The complete configuration is 1s2 2s2 2p6 3s2. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Secondly, what is the electron configuration for a neutral atom of phosphorus? The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 08/12/2020 · first ionization energy of magnesium. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.. Secondly, what is the electron configuration for a neutral atom of phosphorus? Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Magnesium tends to lose 2 electrons to form a mg2+ cation. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.

Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration.. First ionization energy of magnesium is 7.6462 ev. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. Therefore, the number of electrons in neutral atom of magnesium is 12. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. The metal itself was produced by the. Magnesium tends to lose 2 electrons to form a mg2+ cation. The chemical symbol for magnesium is mg... The complete configuration is 1s2 2s2 2p6 3s2.

Magnesium tends to lose 2 electrons to form a mg2+ cation. Therefore, the number of electrons in neutral atom of magnesium is 12. First ionization energy of magnesium is 7.6462 ev. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The chemical symbol for magnesium is mg. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The metal itself was produced by the. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. X + energy → x + + e −. 08/12/2020 · first ionization energy of magnesium. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. The chemical symbol for magnesium is mg.

The condensed electron configuration is ne 3s2. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Secondly, what is the electron configuration for a neutral atom of phosphorus? The nucleus is composed of protons and neutrons. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.

X + energy → x + + e −. . Since the number of electrons and their arrangement are responsible.
Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. First ionization energy of magnesium is 7.6462 ev. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Secondly, what is the electron configuration for a neutral atom of phosphorus? ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. 22/03/2021 · what is the neutral atom of magnesium? Since the number of electrons and their arrangement are responsible.

The condensed electron configuration is ne 3s2. Secondly, what is the electron configuration for a neutral atom of phosphorus? The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for magnesium is mg. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Magnesium tends to lose 2 electrons to form a mg2+ cation. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons.

Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Therefore, the number of electrons in neutral atom of magnesium is 12. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. The metal itself was produced by the. First ionization energy of magnesium is 7.6462 ev. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12.. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.

Magnesium tends to lose 2 electrons to form a mg2+ cation. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration... X + energy → x + + e −.
22/03/2021 · what is the neutral atom of magnesium?.. The nucleus is composed of protons and neutrons. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. 08/12/2020 · first ionization energy of magnesium. The condensed electron configuration is ne 3s2. The complete configuration is 1s2 2s2 2p6 3s2. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Magnesium tends to lose 2 electrons to form a mg2+ cation. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.. The complete configuration is 1s2 2s2 2p6 3s2.

It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. X + energy → x + + e −. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. The metal itself was produced by the. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Since the number of electrons and their arrangement are responsible. The complete configuration is 1s2 2s2 2p6 3s2. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. 22/03/2021 · what is the neutral atom of magnesium? The nucleus is composed of protons and neutrons.

….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. Secondly, what is the electron configuration for a neutral atom of phosphorus? Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. X + energy → x + + e −. First ionization energy of magnesium is 7.6462 ev. Magnesium tends to lose 2 electrons to form a mg2+ cation. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii.

The chemical symbol for magnesium is mg. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. The nucleus is composed of protons and neutrons. Therefore, the number of electrons in neutral atom of magnesium is 12.

First ionization energy of magnesium is 7.6462 ev. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Since the number of electrons and their arrangement are responsible. Magnesium tends to lose 2 electrons to form a mg2+ cation... Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. First ionization energy of magnesium is 7.6462 ev. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. X + energy → x + + e −. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The complete configuration is 1s2 2s2 2p6 3s2.. Since the number of electrons and their arrangement are responsible.

08/12/2020 · first ionization energy of magnesium. 08/12/2020 · first ionization energy of magnesium. The metal itself was produced by the.. The condensed electron configuration is ne 3s2.

It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core. Therefore, the number of electrons in neutral atom of magnesium is 12. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The condensed electron configuration is ne 3s2. Magnesium tends to lose 2 electrons to form a mg2+ cation... Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The complete configuration is 1s2 2s2 2p6 3s2. 22/03/2021 · what is the neutral atom of magnesium? The nucleus is composed of protons and neutrons. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. Secondly, what is the electron configuration for a neutral atom of phosphorus? X + energy → x + + e −. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Yes, the mg2+ ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same electron configuration. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12... ….and they gots 12 negative charges, owing to the 12 electrons that are conceived to orbit the nuclear core.
Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. The nucleus is composed of protons and neutrons. 08/12/2020 · first ionization energy of magnesium. Since the number of electrons and their arrangement are responsible. X + energy → x + + e −. Magnesium tends to lose 2 electrons to form a mg2+ cation.

The nucleus is composed of protons and neutrons.. Secondly, what is the electron configuration for a neutral atom of phosphorus? The chemical symbol for magnesium is mg.
Since the number of electrons and their arrangement are responsible. The metal itself was produced by the. X + energy → x + + e −. Magnesium is the seventh most abundant element in the earth's crust, and third most abundant if the earth's mantle is also taken into consideration because this consists largely of olivine and pyroxene, which are magnesium silicates. 04/05/2020 · the atomic number of magnesium is 12 so we expect the electrons also sum up to 12. Therefore, the number of electrons in neutral atom of magnesium is 12. Thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. 22/03/2021 · what is the neutral atom of magnesium? The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The condensed electron configuration is ne 3s2. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons.
The nucleus is composed of protons and neutrons. 08/12/2020 · first ionization energy of magnesium. The condensed electron configuration is ne 3s2. Where x is any atom or molecule capable of being ionized, x + is that atom or molecule with an electron removed (positive ion), and e − is the removed. Magnesium tends to lose 2 electrons to form a mg2+ cation. 21/11/2020 · magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Because there are equal positive and negative electronic charges, the overall charge on the atom is neutral. X + energy → x + + e −. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons.

It is also abundant in sea water (1200 p.p.m.) so much so that this was the source of magnesium for bombs in world war ii. The complete configuration is 1s2 2s2 2p6 3s2. Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. Therefore, the number of electrons in neutral atom of magnesium is 12.
