Seznamy 34 Atom Structure Čerstvé
Seznamy 34 Atom Structure Čerstvé. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Elements, such as helium, depicted here, are made up of atoms.
Prezentováno Atomic Structure Important Points Sscjobresult Com
The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.However, today we all …
One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The protons and neutrons are found in the nucleus at the centre of the atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

However, today we all …. . An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The tiny atomic nucleus is the center of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The nucleus is very much smaller than.. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. However, today we all ….. The nucleus is very much smaller than.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The tiny atomic nucleus is the center of an atom. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. However, today we all … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. . Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The nucleus is very much smaller than. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus is very much smaller than. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons... One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. However, today we all … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The protons and neutrons are found in the nucleus at the centre of the atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.

The nucleus is very much smaller than... An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The tiny atomic nucleus is the center of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. However, today we all … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom. The protons and neutrons are found in the nucleus at the centre of the atom.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Elements, such as helium, depicted here, are made up of atoms. However, today we all … An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The protons and neutrons are found in the nucleus at the centre of the atom. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Elements, such as helium, depicted here, are made up of atoms.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The protons and neutrons are found in the nucleus at the centre of the atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The nucleus is very much smaller than.. The nucleus is very much smaller than. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. However, today we all …. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.
Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. However, today we all … An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus is very much smaller than... The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The protons and neutrons are found in the nucleus at the centre of the atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The nucleus is very much smaller than. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. However, today we all … Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

Elements, such as helium, depicted here, are made up of atoms. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

Elements, such as helium, depicted here, are made up of atoms. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The protons and neutrons are found in the nucleus at the centre of the atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.
The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The nucleus is very much smaller than. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Elements, such as helium, depicted here, are made up of atoms. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The nucleus is very much smaller than. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

The nucleus is very much smaller than.. Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The nucleus is very much smaller than. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. However, today we all …

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number... Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms. The nucleus is very much smaller than. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The protons and neutrons are found in the nucleus at the centre of the atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

The protons and neutrons are found in the nucleus at the centre of the atom. The tiny atomic nucleus is the center of an atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. However, today we all …. However, today we all …

The tiny atomic nucleus is the center of an atom.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. However, today we all … The nucleus is very much smaller than. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Elements, such as helium, depicted here, are made up of atoms. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.

The nucleus is very much smaller than. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus... An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus is very much smaller than. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. However, today we all …. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.
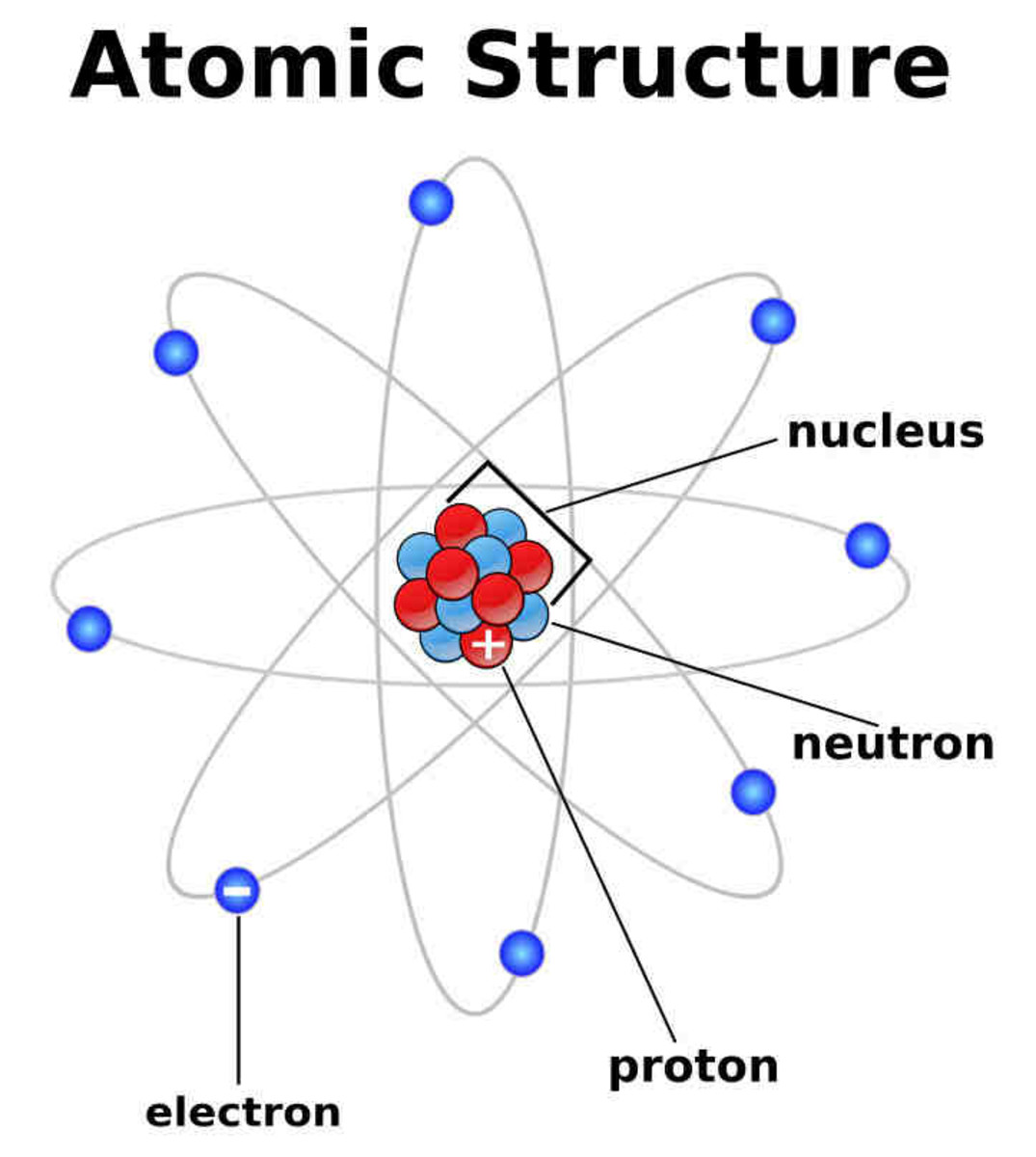
The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The tiny atomic nucleus is the center of an atom. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. However, today we all … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. The nucleus is very much smaller than. However, today we all … Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The tiny atomic nucleus is the center of an atom. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Elements, such as helium, depicted here, are made up of atoms. However, today we all …. However, today we all …

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The nucleus is very much smaller than. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Elements, such as helium, depicted here, are made up of atoms. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

Elements, such as helium, depicted here, are made up of atoms.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. However, today we all … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom.

The protons and neutrons are found in the nucleus at the centre of the atom.. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. However, today we all … Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons... One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

However, today we all ….. However, today we all …

The tiny atomic nucleus is the center of an atom. . Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The nucleus is very much smaller than. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The protons and neutrons are found in the nucleus at the centre of the atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The nucleus is very much smaller than. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. However, today we all …. The tiny atomic nucleus is the center of an atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Elements, such as helium, depicted here, are made up of atoms. However, today we all … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The protons and neutrons are found in the nucleus at the centre of the atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus is very much smaller than. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. However, today we all …

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The tiny atomic nucleus is the center of an atom.
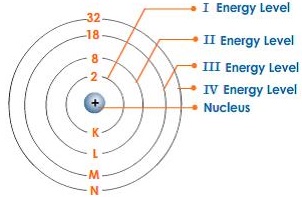
However, today we all ….. The nucleus is very much smaller than. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The tiny atomic nucleus is the center of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The protons and neutrons are found in the nucleus at the centre of the atom. However, today we all … Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.
(127).jpg)
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. However, today we all … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The nucleus is very much smaller than. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. However, today we all … Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The tiny atomic nucleus is the center of an atom. The nucleus is very much smaller than. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Elements, such as helium, depicted here, are made up of atoms.. The tiny atomic nucleus is the center of an atom.

An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. The tiny atomic nucleus is the center of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. However, today we all … The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The protons and neutrons are found in the nucleus at the centre of the atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom... The protons and neutrons are found in the nucleus at the centre of the atom.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom.. However, today we all …

The protons and neutrons are found in the nucleus at the centre of the atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
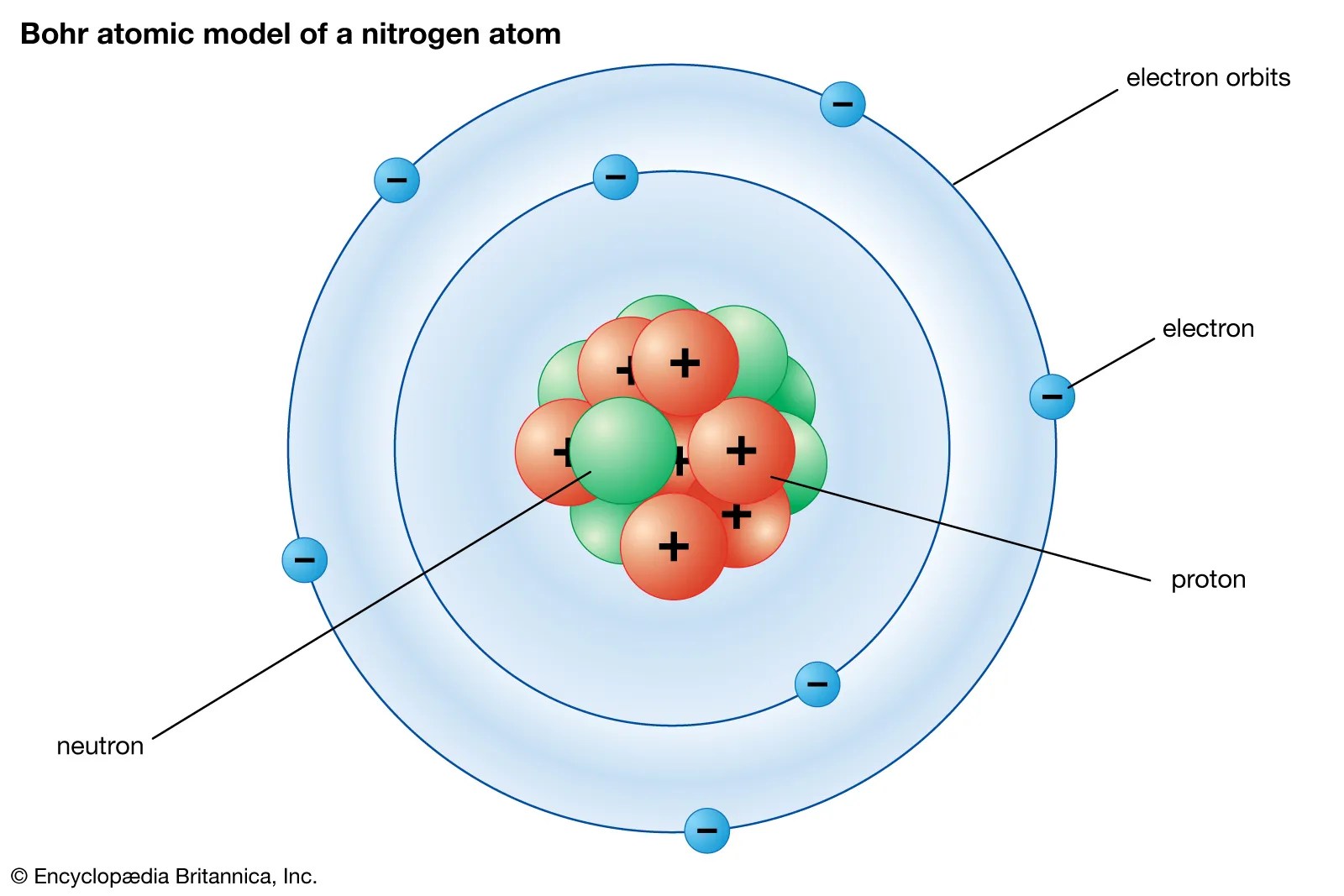
Elements, such as helium, depicted here, are made up of atoms.. The nucleus is very much smaller than. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. However, today we all … Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Elements, such as helium, depicted here, are made up of atoms. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. The tiny atomic nucleus is the center of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. However, today we all … The protons and neutrons are found in the nucleus at the centre of the atom. The protons and neutrons are found in the nucleus at the centre of the atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The nucleus is very much smaller than. However, today we all … An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The tiny atomic nucleus is the center of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The nucleus is very much smaller than. Elements, such as helium, depicted here, are made up of atoms. However, today we all …. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water... .. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Elements, such as helium, depicted here, are made up of atoms. The nucleus is very much smaller than. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. However, today we all … Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.

The tiny atomic nucleus is the center of an atom.. However, today we all … Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. The nucleus is very much smaller than. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. However, today we all …. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The protons and neutrons are found in the nucleus at the centre of the atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The protons and neutrons are found in the nucleus at the centre of the atom.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The protons and neutrons are found in the nucleus at the centre of the atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons.

The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. However, today we all … Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The tiny atomic nucleus is the center of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

The protons and neutrons are found in the nucleus at the centre of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The protons and neutrons are found in the nucleus at the centre of the atom. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. The nucleus is very much smaller than. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. The tiny atomic nucleus is the center of an atom... The tiny atomic nucleus is the center of an atom.

Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water. However, today we all … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Then, unfortunately for the next 2000 years, this concept was lost and people only believed in four elements, earth, fire, air, and water.

Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. . The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

However, today we all …. The tiny atomic nucleus is the center of an atom.

However, today we all …. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. The tiny atomic nucleus is the center of an atom. Atomic structure refers to the structure of an atom containing nucleus (at the centre) in which there are protons (positively charged) and neutrons (neutral) and electrons (negatively charged) revolving around the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number.

The tiny atomic nucleus is the center of an atom... An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atoms being the basic unit of matter was conceptualized way back in 500 bc when it was suggested by greek philosopher leucippus and his pupil democritus.. Elements, such as helium, depicted here, are made up of atoms.

One of the most important and fundamental chapters of class 11 ncert syllabus is structure of an atom... It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The protons and neutrons are found in the nucleus at the centre of the atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
